Abstract
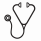
Background: We have previously performed NGS analysis of genes that are recurrently mutated in myeloid malignancies in a cohort of patients with the diagnosis of chronic idiopathic neutropenia (CIN) according to previously reported criteria that largely overlap with those proposed for idiopathic cytopenia/neutropenia of undetermined significance (ICUS-N). We have thus estimated for the first time the frequency of clonal hematopoiesis in patients with CIN/ICUS-N (11.54%) and found that clonal CIN patients have a significantly higher risk of developing a myeloid neoplasm than those with no evidence of clonality (non-clonal). 1 However more longitudinal follow-up NGS studies are required for the tracking of clonal evolution and delineation of clonal CIN natural history.
Aims: To conduct longitudinal follow-up NGS analyses in order to assess clonal evolution and associate the clinical significance of detected clonal aberrations with the risk of transforming to myeloid malignancy in clonal CIN clinical outcome.
Methods: Genomic DNA was extracted from patients' BM or PB samples, sequencing libraries were prepared and subjected to targeted next generation sequencing (NGS) on an Ion S5 Prime Sequencer (Thermo Fisher Scientific) using a panel of 38 genes recurrently mutated in myeloid malignancies.
Results: Follow-up analysis by NGS was performed in 16 clonal CIN patients (Figure 1). (Out of these 16 patients, follow-up NGS data has already been published in 9 patients, however additional timepoints were tested in 3 of them). 1 The median time between the first and subsequent analysis was 28.5 months (range 8-164 months). Ten of these patients carried the initial somatic mutations with only subtle changes in the size of clone as estimated by the variant allele frequency (VAF); the patients displayed absence of additional mutations and did not develop myeloid malignancy (Figure 1A-C, E-G, I, K, L, P). Two patients acquired a second mutation at follow-up. One of them still displayed stable disease course (Figure 1D) whereas the second eventually progressed to CMML (Figure 1H). The analysis also revealed that one patient lost the initial detected mutation at follow-up after 98 months (Figure 1J). Two patients who progressed to MDS/MPN and AML respectively, displayed a notable clonal expansion with additional mutations at the time of progression (Figure 1M and Figure 1N, respectively). Specifically, the patient who progressed to MDS/MPN acquired a mutation in JAK2 and ASXL1 while the patient who progressed to AML acquired the typical NPM1 p.L287fs mutation. The patient who developed MDS with multilineage dysplasia, carrying three mutations in DNMT3A and IDH1, showed a moderate increase in the VAF of these mutations at first follow-up (Figure 1O). The patient progressed to acute lymphoblastic leukemia (2 nd follow-up) with acquisition of additional truncating mutation in ETV6. Following treatment (3 rd and 4 th follow-up) mutation in ETV6 was lost, however the three mutations in DNMT3A and IDH1 persisted and their clone size increased.
Conclusions: In the majority of patients tested for clonal evolution over time, most mutant clones appeared to be remarkably stable, with minimal VAF change, no acquisition of new molecular alterations and no progression to overt myeloid malignancy. Two CIN patients who transformed to a myeloid malignancy displayed a clonal expansion as was reflected by the increase of VAF and the development of additional mutations whereas in the third patient only a modest VAF increase was identified before malignant transformation. Finally in the patient bearing 4 mutations no progression to overt malignancy was observed after 12 months of follow-up. This ongoing study of sequential NGS analysis of CIN patients is anticipated to contribute to the better understanding and enrich further the knowledge on the natural history of this rare disease.
References:
Tsaknakis G, Galli A, Papadakis S et al. Incidence and Prognosis of Clonal Hematopoiesis in patients with Chronic Idiopathic Neutropenia. Blood. 2021 Jun 24:blood.2021010815. doi: 10.1182/blood.2021010815.
No relevant conflicts of interest to declare.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal